Remarkable results achieved on Innovative Immunotherapy Drugs Strengthen the cooperation between Poly and US innovative pharmaceutical company Imunon
Hainan Poly Pharm. Co., Ltd. has launched a deep cooperation with Imunon, Inc., a leading innovative pharmaceutical company in the United States to assist CDMO in developing a new immunotherapy drug for the treatment of ovarian cancer.
Ovarian cancer refers to a malignant tumor that grows on the ovaries, with over 300,000 new confirmed cases worldwide and over 60,000 new confirmed cases in China each year, ranking first in the world.
(Annual new confirmed cases of ovarian cancer)
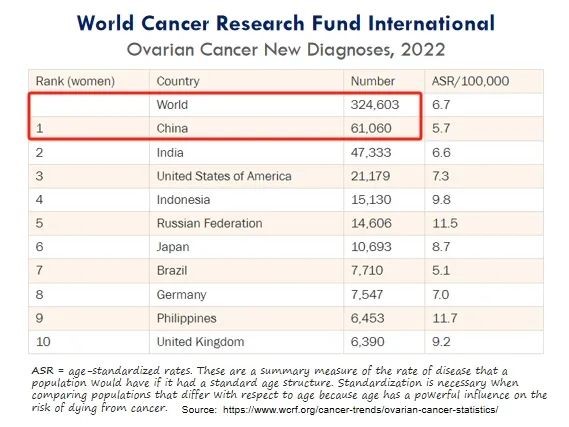
Due to the lack of symptoms and no specific symptoms in the early stages of ovarian cancer, plus the limit of screening, the early diagnosis is difficult, and 80% of patients are already in the advanced stage (III/IV) at the time of diagnosis. However, the treatment efficacy in the advanced cases is poor, with mortality rate over 60% within 5 years, which is higher than the sum of cervical cancer and endometrial cancer, ranking first among gynecological cancers.
For decades, the standard treatment plan for ovarian cancer has been stagnant, with surgery and chemotherapy being the main treatment. In terms of surgical treatment, patients in the advanced stages should undergo tumor cell reduction surgery to remove visible tumors to the greatest extent possible. In terms of chemotherapy, paclitaxel combined with carboplatin is the preferred first-line chemotherapy. In addition, targeted therapy for maintenance therapy (anti angiogenic drugs: bevacizumab, PARP inhibitors: olaparib, niraparib) also provides more options for the treatment of ovarian cancer.
Despite these efforts, the improvement in overall survival rate (OS) remains difficult to achieve.
The new immunotherapy drugs developed by innovative pharmaceutical companies in the United States have the potential to break through today's standard front line treatment protocols and become the first and only immunotherapy for ovarian cancer.
Interleukin-12 (IL-12) is the core of the immune response against cancer, which can activate the immune system to attack tumors. The nano particle characteristics of the new immunotherapy drug IMNN-001 developed by Imunon enables cell transfection and can locally and sustainably deliver IL-12 to support the immune system in combating ovarian cancer, avoiding systemic toxicity observed with earlier attempts in the industry to harness IL-12.
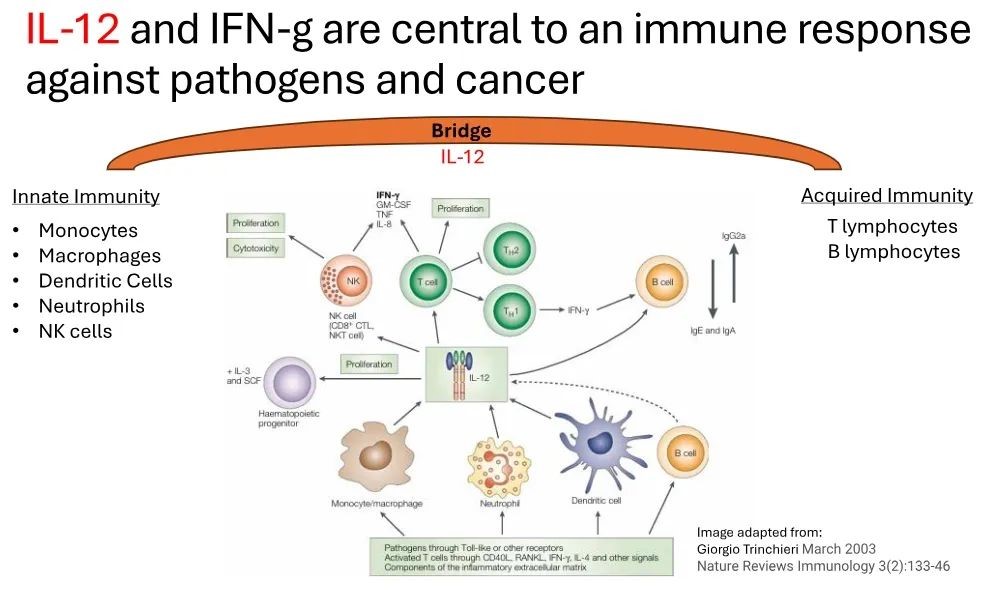
In previous cooperation, Poly Pharm. has provided high-quality clinical batch samples to its American partners, which have been used in early clinical trials and achieved excellent results in early stage clinical trials.
Based on the high recognition and trust of each other's professional abilities, both parties have further expanded the scope of cooperation to achieve greater synergies. Poly Pharm. will continue to improve the process for the new drug and the subsequent clinical sample production.
Phase II clinical trial results reach industry-leading leve
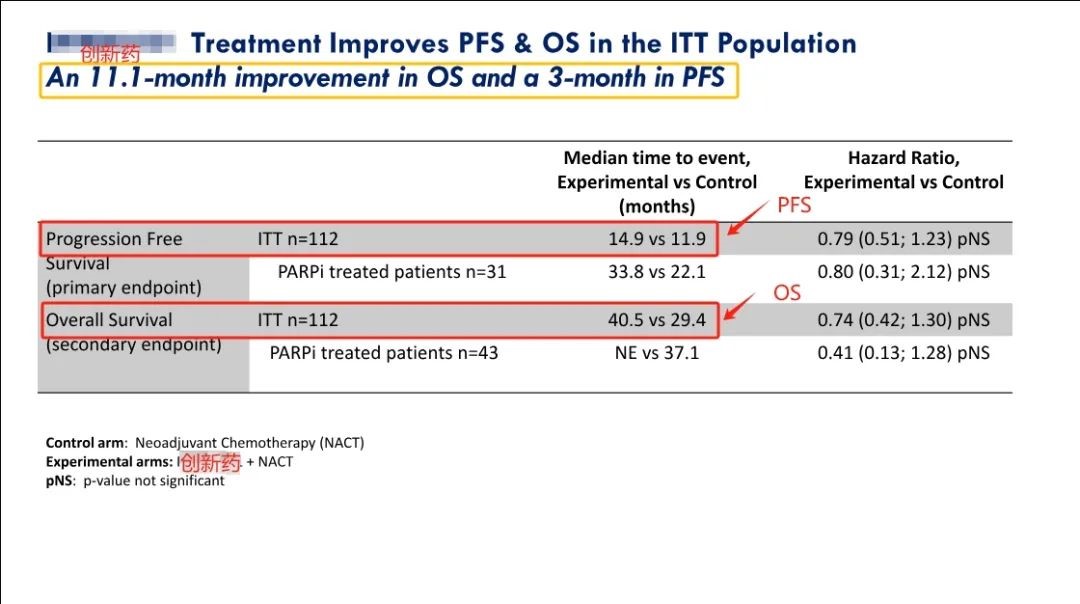
Recently, the Phase II clinical trial results of IMNN-001 this innovative drug have been announced which shows better performance than the standard treatment in the treatment group and if successful in Phase 3 is expected to replace the standard treatment as a new first-line treatment regimen. The research results are as follows:
1) Compared with standard treatment alone, the median overall survival (OS) of the intention to treat population (ITT) increased by 11.1 months. Among them, for the population receiving PARP inhibitor treatment, the median OS was not yet reached in women receiving IMNN-001 treatment, with some women approaching the 5-year mark. . Compared to the standard treatment group, the improvement in OS with IMNN-001 in these women was 144%.
2) The median OS of patients receiving ≥3 doses of new immunotherapy drugs increased by 15.7 months.
3) Compared with standard treatment alone, PFS (progression free survival) showed a 3-month improvement.
Given the clinical trial results mentioned above, both parties are confident in the Phase III clinical trial and commercialization.



(Visit Hangzhou Site (Zhejiang) )



(Visit Haikou Site (Hainan) )
In addition, once the registration clinical trials are successfully completed and marketing authorization obtained from relevant regulatory authorities, Poly Pharm. will be responsible for the commercial production of the drug to ensure its entry into the market and meet the needs of many patients. Through this collaboration, both parties will jointly promote the successful development and market promotion of the new drug project, bringing good news to more patients.
The drug is expected to be submitted in China in the future, and both parties will further cooperate to jointly explore the Chinese market. At the same time, Poly Pharm. will also strive to promote the landing of the drug in Hainan Boao Lecheng International Medical Tourism Pioneer Zone (Lecheng Pioneer Zone, located in Boao, a small town in the southeast of Hainan Free Trade Port, has a unique licensing policy: it allows the import and use of drugs and medical devices that have been listed overseas but not approved for registration in China, so that the citizen can experience international cutting-edge medical services without leaving the city), and introduce innovative drugs to China to benefit domestic patients.

Poly Pharmaceuticals was established in Haikou in 1992 and is a pioneer enterprise in the internationalization of Chinese pharma-ceutical preparations and a demonstration enterprise for intelligent manufacturing by the Ministry of Industry and Information Technology of China. It has been included by the Ministry of Industry and Information Technology in the key projects for the industrial transformation and upgrading of "Made in China 2025" for children's medicines. In 2023, Hainan Poly passed the "AEO" advanced certification of customs.
Previously, Hainan Poly and its subsidiaries, Zhejiang Poly and Anhui Poly, have also successfully passed on-site audits by the U.S. FDA and the European Union EMA. As a pioneer enterprise in the internationa-lization of Chinese pharmaceutical pre-parations, Poly Pharmaceuticals has adhered to high global quality standards for many years. It is one of the few platforms in China for the research and development and production of APIs and injectables, and it is also one of the few high-quality suppliers that have been approved by regulatory agencies such as the FDA in the United States, the NMPA in China, and the EMA in the European Union for APIs, key excipients, pharmaceutical preparations, and GMP intermediates CMO/CDMO.

 Home
Home

